We need to know the root mean square speed of Cl₂ molecule.
The root mean square speed of Cl₂ molecule is:3.67 X 10⁴ cm/sec.
Average kinetic energy=
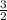 RT, where R=Molar gas constant and T=absolute temperature in Kelvin.
RT, where R=Molar gas constant and T=absolute temperature in Kelvin.
Given, average kinetic energy= 4790 J/mol, R= 8.314 J/mol/K
So, T=
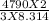
T=384.09 K
Root mean square velocity=

For, Cl₂ molecule, M= 71 g/mol, T=384.09 K (as condition is same as previous)
So, Root mean square velocity=
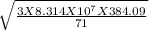 =3.67 X 10⁴ cm/sec
=3.67 X 10⁴ cm/sec
Hence, Root mean square velocity= 3.67 X 10⁴ cm/sec.