Answer : The polyatomic ion with the formula
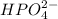 is called hydrogen phsophate ions.
is called hydrogen phsophate ions.
Explanation :
The rules for naming the ionic compounds with polyatomic ion is given as :
- The positive ion (cation) is written first.
- The negative ion (anion) is written next.
- The suffix is added at the end of the negative ion (anion). The suffix used is '-ate'.
In
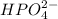 , hydrogen ion combine with phosphate ion to give hydrogen phosphate ion.
, hydrogen ion combine with phosphate ion to give hydrogen phosphate ion.
Hence, the polyatomic ion with the formula
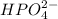 is called hydrogen phsophate ions.
is called hydrogen phsophate ions.