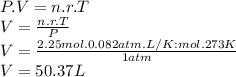Answer:
the volume that occupies 2.25 moles of neon gas in STP conditions is of 50.37L
Step-by-step explanation:
the gas is in STP conditions, this means that it is in standard conditions of temperature and pressure
For standard conditions the pressure has a value of 1atm and the temperature has a value of 273 K
To calculate what volume 2.25 moles of neon gas occupy we use the equation of ideal gases
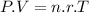
r (ideal gas constant) =
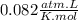
we clear the volume