Answer: Option (A) is the correct answer.
Explanation:
A covelent bond is a bond which occurs when electrons are shared between between atoms.
For example,
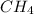 is a covalent compound because valency of carbon is 4 and valency of hydrogen is 1. So, when both carbon and hydrogen comes in contact with each other then there occurs sharing of electrons.
is a covalent compound because valency of carbon is 4 and valency of hydrogen is 1. So, when both carbon and hydrogen comes in contact with each other then there occurs sharing of electrons.
Thus, we can conclude that in a covalent bond, electrons that are shared between atoms holds the atoms together.