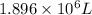Answer : The volume of the air in the balloon after it heated is,
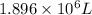
Solution : Given,
Initial volume of air balloon =
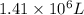
Initial temperature of air balloon =
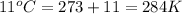
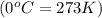
Final temperature of air balloon =
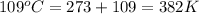
According to the Charles' law, the volume of an ideal gas is directly proportional to the temperature of the gas at constant pressure.
It is represented as,
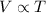
or,
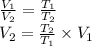
where,
 = initial volume of air balloon
= initial volume of air balloon
 = final volume of air balloon
= final volume of air balloon
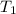 = initial temperature of air balloon
= initial temperature of air balloon
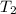 = final temperature of air balloon
= final temperature of air balloon
Now put all the given values in the above formula, we get the final volume of air balloon.
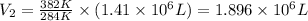
Therefore, the volume of the air in the balloon after it heated is,