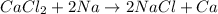Answer: The correct answer is
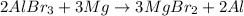 and
and
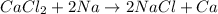
Step-by-step explanation:
Single displacement reactions are the reactions in which a more reactive element replaces a less reactive element from its chemical reaction.
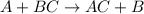
A is more reactive element than reactive B.
The reactivity of metals is judged by the series known as reactivity series. Elements lying above in the series are more reactive than the elements lying below in the series.
For the given chemical reactions:
Reaction 1:
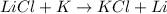
Potassium lies below in the series than lithium. Thus, it will not replace lithium from its chemical reaction. It is not considered as a single displacement reaction.
Reaction 2:
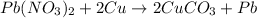
Copper lies below in the series than lead. Thus, it will not replace lead from its chemical reaction. It is not considered as a single displacement reaction.
Reaction 3:
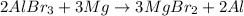
Magnesium lies above in the series than aluminium. Thus, it will easily replace magnesium from its chemical reaction. It is considered as a single displacement reaction.
Reaction 4:
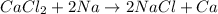
Sodium lies above in the series than calcium. Thus, it will easily replace calcium from its chemical reaction. It is considered as a single displacement reaction.
Hence, the correct answer is
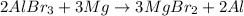 and
and