The give compound is a sparingly soluble salt,
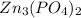 . The solubility equilibrium for this compound can be represented as:
. The solubility equilibrium for this compound can be represented as:
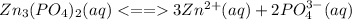
Initial(M) - 0 0
Change(M) - +3x +2x
Equilibrium(M) - 3x 2x
Solubility product expression for this equilibrium will be:
![K_(sp)=[Zn^(2+)]^(3)[PO_(4)^(3-)]^(2)](https://img.qammunity.org/2019/formulas/chemistry/college/4uulbf2f1pkqgwx377bw42z4d96f4lhzuz.png)
=
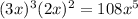
Where x will be the molar solubility of the compound
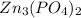 at that given temperature.
at that given temperature.