Answer:
0.11mol/dm³
Step-by-step explanation:
The reaction expression is given as:
HCl + NaOH → NaCl + H₂O
Volume of acid = 25cm³ = 0.025dm³
Volume of base = 18.4cm³ = 0.0184dm³
Concentration of base = 0.15mol/dm³
Solution:
The concentration of hydrochloric acid = ?
To solve this problem, let us first find the number of moles of the base;
Number of moles = concentration x volume
Number of moles = 0.15mol/dm³ x 0.0184dm³ = 0.00276mol
From the balanced reaction equation;
1 mole of NaOH will combine with 1 mole of HCl
Therefore, 0.00276mol of the base will combine with 0.00276mol of HCl
So;
Concentration of acid =
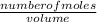 =
=
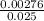 = 0.11mol/dm³
= 0.11mol/dm³