Answer: C.
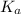 would increase and
would increase and
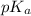 would decrease.
would decrease.
Step-by-step explanation:
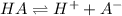
initially conc. c 0 0
At eqm.
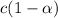
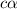
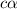
The expression for dissociation constant is,
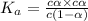
for weak acid,
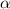 is very very small , the expression will be,
is very very small , the expression will be,
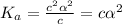
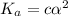 As
As
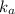 is directly proportional to concentration, the value of
is directly proportional to concentration, the value of
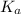 would increase on increasing the concentration.
would increase on increasing the concentration.
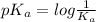
Thus if
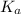 would increase,
would increase,
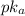 would decrease.
would decrease.