Answer: The percentage composition of hydrogen in the given compound is 15%.
Step-by-step explanation:
To calculate the percentage composition of hydrogen in a compound, we use the formula:
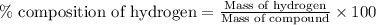
Where,
Mass of hydrogen = 41.4 grams
Mass of compound = 276 grams
Putting values in above equation, we get:
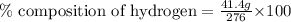
Percentage composition of hydrogen in a given compound = 15%