Answer: There are 3 triple points in the given phase diagram.
Explanation: Triple point is defined as the point at which the temperature and pressure of the 3 phases are in equilibrium with each other.
In the given phase diagram, there are three points which is satisfying this definition.
Point A: At this point The monoclinic form and rhombic form have the same vapor pressure. Hence,
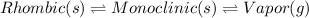
Point B: At this point, the monoclinic form starts to melt. Hence,
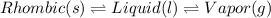
Point C: At this point, the rhombic form, monolinic form and liquid is in equilibrium. Hence,
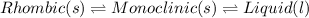
Hence, this system has 3 triple points.