Solution :
Given :
length of silver cube = 2.28 cm
length of gold cube = 2.75 cm
Initial temperature = 82.8°C
Volume of silver cube is
Volume
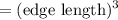
=
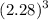
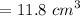
mass of silver cube
Mass,
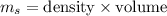
= 10.5 x 11.8
= 123.9 g
Similarly, the volume of gold cube is
Volume
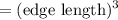
=
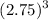
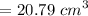
mass of gold cube
Mass,
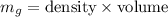
= 19.3 x 20.79
= 401.247 g
Now
heat lost by silver and gold cube = heat gained by water
∴
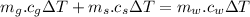
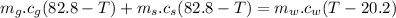
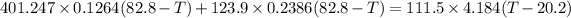
Now solving the equation
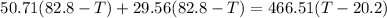
Final temperature, T = 31.27°C