Answer:
135.92Pa
Step-by-step explanation:
This question requires using the Combined Gas Law,
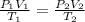 , wherein the P1, V1, and T1 are the initial gas conditions and P2, V2, and T2 are conditions after. We are given all three of the initial conditions and two out of the three conditions after the changes, which means we can solve for the missing variable in the equation.
, wherein the P1, V1, and T1 are the initial gas conditions and P2, V2, and T2 are conditions after. We are given all three of the initial conditions and two out of the three conditions after the changes, which means we can solve for the missing variable in the equation.
Here are the steps for solving the equation:
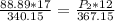
multiply the numerator of the initial side of the equation
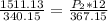
divide 1511.13 by 340.15
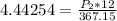
multiply both sides of the equation by 367.15
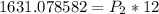
divide both sides by 12
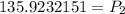
If you round this to the nearest hundreds place you get a pressure of 135.92Pa