Answer:
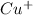 ion size is larger than
ion size is larger than
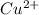 ion.
ion.
This is because
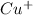 has one more electron than
has one more electron than
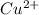 .
.
Step-by-step explanation:
Cations: They are formed when an atom looses its valence electrons. They are positive ions.
For positive ions, the removal of electron increases the nuclear charge for an outermost electron because the outermost electrons are more strongly attracted by the nucleus. So, the effective nuclear charge increases for cations and with that size of the ion also decreases.
As the positive charge on an ion increases the effective nuclear charges also increases which pulls remaining electrons present in the valence shell more closer towards the nucleus. Due to this size of an ion further decreases.
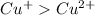
Also number of electrons in
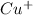 ions are more than that of the
ions are more than that of the
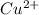 ions.
ions.
So, that is
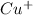 ion size is larger than
ion size is larger than
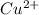 ion.
ion.