Answer:
The ΔH of the reaction when 48.7 grams of ZnS reacts with oxygen is -55.23 kJ.
Step-by-step explanation:
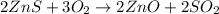
Amount of ZnS = 48.7 grams
Molecular mass of ZnS = 97.474 g/mol

Moles of ZnS.:
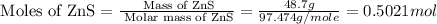
According to reaction, 2 moles of ZnS gives energy = -220 kJ
So, 0.5021 moles of ZnS gives energy :
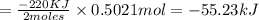
The ΔH of the reaction when 48.7 grams of ZnS reacts with oxygen is -55.23 kJ.