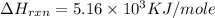Answer :
When sucrose is oxidized by the oxygen gas to produces carbon dioxide and water as a product.
The balanced thermochemical equation for this reaction is,
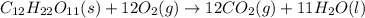
- Enthalpy of reaction : It is defined as the amount of energy or heat absorbed or released per mole in the reaction.
The
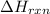 is represented in scientific notation as,
is represented in scientific notation as,