Answer:
Magnesium
0.003mole
Step-by-step explanation:
The problem here entails we find the metal in the carbonate.
For group 2 member, let the metal = X;
The carbonate is XCO₃;
If we sum the atomic mass of the elements in the metal carbonate, we should arrive at 84g/mol
Atomic mass of C = 12g/mol
O = 16g/mol
Atomic mass of X + 12 + 3(16) = 84
Atomic mass of X = 84 - 60 = 24g/mol
The element with atomic mass of 24g is Magnesium
B.
Number of moles in 0.3g of CaCO₃:
Molar mass of CaCO₃ = 40 + 12 + 3(16) = 100g/mol
Number of moles =
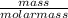
Number of moles =
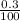 = 0.003mole
= 0.003mole