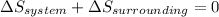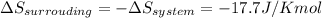Answer: The entropy change of the surroundings will be -17.7 J/K mol.
Explanation: The enthalpy of vapourization for 1 mole of acetone is 31.3 kJ/mol
Amount of Acetone given = 10.8 g
Number of moles is calculated by using the formula:
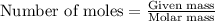
Molar mass of acetone = 58 g/mol
Number of moles =
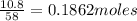
If 1 mole of acetone has 32.3 kJ/mol of enthalpy, then
0.1862 moles will have =
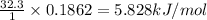
To calculate the entropy change for the system, we use the formula:
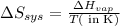
Temperature = 56.2°C = (273 + 56.2)K = 329.2K
Putting values in above equation, we get
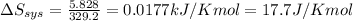 (Conversion Factor: 1 kJ = 1000J)
(Conversion Factor: 1 kJ = 1000J)
At Boiling point, the liquid phase and gaseous phase of acetone are in equilibrium. Hence,