Answer: 0.8541 grams of HCl will be required.
Step-by-step explanation: Moles can be calculated by using the formula:
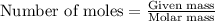
Given mass of
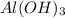 = 0.610 g
= 0.610 g
Molar mass of
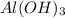 = 78 g/mol
= 78 g/mol
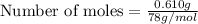
Number of moles of
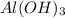 = 0.0078 moles
= 0.0078 moles
The reaction between
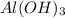 and HCl is a type of neutralization reaction because here acid and base are reacting to form an salt and also releases water.
and HCl is a type of neutralization reaction because here acid and base are reacting to form an salt and also releases water.
Chemical equation for the above reaction follows:
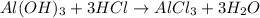
By Stoichiometry,
1 mole of
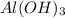 reacts with 3 moles of HCl
reacts with 3 moles of HCl
So, 0.0078 moles of
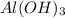 will react with
will react with
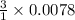 = 0.0234 moles
= 0.0234 moles
Mass of HCl is calculated by using the mole formula, we get
Molar mass of HCl = 36.5 g/mol
Putting values in the equation, we get
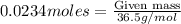
Mass of HCl required will be = 0.8541 grams