This hypothetical process would produce actinium-230.
Step-by-step explanation
An alpha decay reduces the atomic number of a nucleus by two and its mass number by four.
There are two types of beta decay: beta minus β⁻ and beta plus β⁺.
The mass number of a nucleus stays the same in either process. In β⁻ decay, the atomic number increases by one. An electron e⁻ is produced. In β⁺ decay, the atomic number decreases by one. A positron e⁺ is produced. Positrons are antiparticles of electrons.
β⁻ are more common than β⁺ in decays involving uranium. Assuming that the "beta decay" here refers to β⁻ decay.
Gamma decays do not influence the atomic or mass number of a nucleus.
Uranium has an atomic number of 92. 238 is the mass number of this particular isotope. The hypothetical product would have an atomic number of 92 - 2 ⨯ 2 + 1 = 89. Actinium has atomic number 89. As a result, the product is an isotope of actinium. The mass number of this hypothetical isotope would be 238 - 2 ⨯ 4 = 230. Therefore, actinium-230 is produced.
The overall nuclear reaction would involve five different particles. On the reactant side, there is
On the product side, there are
- one actinium-230 atom,
- two alpha particles (a.k.a. helium-4 nuclei),
- one electron, and
- one gamma particle (a.k.a. photon).
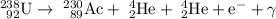
Consider: what would be the products if the nucleus undergoes a β⁺ decay instead?