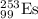Answer: 1) The nuclide formed from the alpha decay of
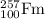 is
is
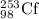
2) The nuclide formed from the beta-minus decay of
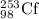 is
is
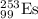
Explanation:
1) For alpha-decay
In this process, alpha particle is released.
Equation follows:
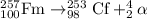
The nuclide formed is
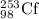
2) For beta-minus decay
In this process, a neutron is converted into a proton and an electron.
Equation follows:

The nuclide formed is