Answer : The correct option is, 4
Explanation :
Balanced chemical reaction : In the balanced chemical reaction the number of individual atoms present of reactant side must be equal to the product side.
The given balanced reaction will be,
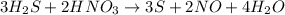
By stoichiometry we can say that, 3 moles of hydrogen sulfide react with 2 moles of nitric acid to give 3 moles of sulfur, 2 moles of nitrogen oxide and 4 moles of water as products.
The coefficient for
 is, 4
is, 4
Therefore, the correct option is, 4