Answer : The correct option is
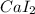 .
.
Explanation :
Ionic compound : It consist of a metal and non-metal. It is made up of ions that gains or lose electrons and giving them a net positive and negative charge.
Metals have tendency to lose electrons easily, so they become cation which have a positive charge and non-metal have tendency to gain electrons, so they become anion which have a negative charge.
As we know that the Calcium forms ions with a charge of (+2) and Iodine forms ions with a charge of (-1).
Two iodide ion will require to combine with one calcium ion for a neutral compound.
Therefore, the correct representation of an ionic compound of Calcium and Iodide ions is
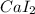 .
.
The image is shown below.