Answer: X = 2 and Y = 1.
Step-by-step explanation:
If Potassium ions and Selenide ions combine to form Potassium Selenide then according to their respective charges we do the cross multiplication in order to form the compound.
Charge on potassium ion is +1, and charge on selenide ion is -2. Therefore, by cross multiplication we need 2 atoms of potassium and 1 atom of selenide to form Potassium Selenide.
Therefore, the formula of compound will be
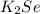 .
.
Thus, we can conclude that X = 2 and Y = 1.