Answer: The enthalpy of formation of AgCl is -127.3 kJ/mol
Step-by-step explanation:
To calculate the number of moles, we use the equation:
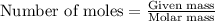
Given mass of silver chloride = 2.21 g
Molar mass of silver chloride = 143.32 g/mol
Putting values in above equation, we get:
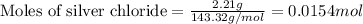
Sign convention of heat:
When heat is absorbed, the sign of heat is taken to be positive and when heat is released, the sign of heat is taken to be negative.
To calculate the enthalpy change of formation of AgCl, we use the equation:
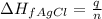
where,
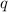 = amount of heat released = -1.96 kJ
= amount of heat released = -1.96 kJ
n = number of moles of AgCl = 0.0154 moles
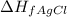 = enthalpy of formation of silver chloride
= enthalpy of formation of silver chloride
Putting values in above equation, we get:
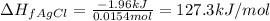
Hence, the enthalpy of formation of AgCl is -127.3 kJ/mol