Answer : The ratio of effusion rates for helium and argon is 3.2 : 1
Solution : Given,
Molar mass of Helium = 4 g/mole
Molar mass of Argon = 40 g/mole
Rate of effusion : It is defined as the rate of effusion of a gas is inversely proportional to the square root of the molar mass of the gas.
Formula used :
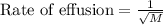
where, M is the molar mass.
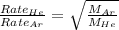
Now put all the given values in this expression, we get
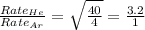
Therefore, the ratio of effusion rates for helium and argon is 3.2 : 1