Answer: Option 'B' is correct.
Explanation:
Let the initial concentration be
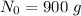
Let concentration after t= 90 hours be N
Since we have given that Sodium-24 has a half life of 15 hours i.e.
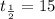
So, According to question,

As we know that
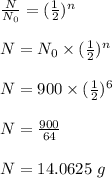
Hence, Option 'B' is correct.