Answer : The correct option is, (C) 2, 4 and 5.
Explanation :
Combustion reaction : It is a type of reaction in which a hydrocarbon react with an oxygen molecule to give carbon dioxide, water as a product.
For example : Methane react with oxygen to give carbon dioxide and water.
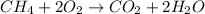
In the given list of chemical substances,
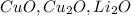 are in oxide form. They can not be both reactant and product of a single combustion reaction.
are in oxide form. They can not be both reactant and product of a single combustion reaction.
In the given list,
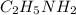 is the only hydrocarbon which shows a combustion reaction. That means
is the only hydrocarbon which shows a combustion reaction. That means
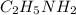 react with
react with
 to give
to give
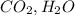 and
and
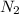 as a product.
as a product.
The balanced combustion reaction of
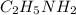 is,
is,
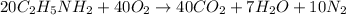
Therefore, the correct answer is, (C) 2, 4, and 5.