Answer: The element Na (Sodium) is getting oxidized and Hydrogen is getting reduced.
Explanation:
Oxidation reactions are the reactions in which addition of oxygen takes place.
Reduction reactions are the reactions in which loss of oxygen takes place.
For a given reaction:
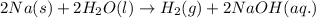
Sodium is getting oxidized because there is an addition of reaction with that element.
Hydrogen is getting reduced because there is a removal of oxygen with that element.