Explanation: The given reaction between is a type of neutralization reaction.
Neutralization reaction is a type of chemical reaction in which an acid reacts with a base to form a salt and water as the products.
General equation for Neutralization reaction is:

For the given reaction.
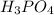 is an acid.
is an acid.
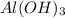 is a base.
is a base.
Reaction between these two is given by:
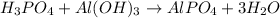
Phosphoric acid and aluminium hydroxide reacts to produce aluminium phosphate salt and water as products.