Answer: The correct option is The properties of a noble gas.
Explanation: There are 7 periods in the periodic table.
The last element of each period are Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), Radon (Rn) and Ununoctium (Uuo).
- The electronic configuration for Helium is
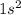 . For He, The outermost electrons are 2.
. For He, The outermost electrons are 2.
- The electronic configuration for all the other elements is
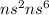 ( where, n = 2, 3, 4, 5, 6 and 7 respectively). For all the other gases, the outermost electrons are 8.
( where, n = 2, 3, 4, 5, 6 and 7 respectively). For all the other gases, the outermost electrons are 8.
All these elements have stable electronic configuration and are not reactive in nature. Hence, they are considered as noble gases.
Therefore, the last element of each period always have the properties of a noble gas.