Answer: The correct answer is coefficients.
Step-by-step explanation:
Coefficients are defined as the numbers which are written in front of the atoms or molecules in a chemical reaction. They represent the number of moles of substance in a chemical reaction.
Subscript numbers are defined as the number of atoms of each element present in a compound.
Superscript numbers are defined as the charges of ions reacting in a chemical reaction.
For Example: Dissociation of sodium carbonate into its ions, the equation follows:
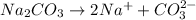
The number '2' on the left side written in the subscript of sodium ion represents the number of sodium atoms.
The number '2' on right side written in front of sodium ion represents the number of moles of sodium ions.
The number '(2-)' on right side written in the superscript of carbonate ion represents the charge on carbonate ion.
Hence, the correct answer is coefficients.