Step-by-step explanation:
The chemical reactions that occur during burning and rusting have the process of oxidation in common.
As oxidation is a process where there is loss of electrons or addition of oxygen atom.
For example, the chemical reaction occurring in rusting is as follows.
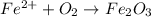
Here, Fe losses an electron and oxygen gains that one electron as a result, there is formation of iron (III) oxide.
In a burning reaction, the process of oxidation also occurs.
For example, the chemical reaction occurring when carbon is burnt in oxygen is as follows.
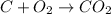
In this process, addition of oxygen displays that oxidation is taking place.