Answer: a)
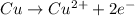 : anode
: anode
b.
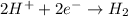 : cathode
: cathode
c.
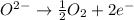 : anode
: anode
Step-by-step explanation:
Electrochemical cell is a device which converts chemical energy into electrical energy. It consist of two electrodes, anode and cathode.
Oxidation i.e. loss of electrons , which results in an increase in oxidation number occurs over anode.
Reduction i.e. gain of electrons, which results in decrease in oxidation number occurs over cathode.
a.
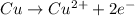 : oxidation : anode
: oxidation : anode
b.
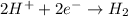 : reduction : cathode
: reduction : cathode
c.
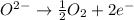 : oxidation : anode
: oxidation : anode