Answer: Molar masses of KBr and NaI
Step-by-step explanation:
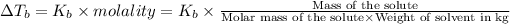
From the the above formula ,we can say that
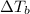 is inversely related to molar mass of the compound. Lower the value of molar mass more will be the value of
is inversely related to molar mass of the compound. Lower the value of molar mass more will be the value of
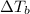 which means more will be the lowering in the freezing temperature of that solution and vice-versa.
which means more will be the lowering in the freezing temperature of that solution and vice-versa.
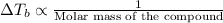
For KBr
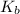 for water is = 0.59 K kg/mol
for water is = 0.59 K kg/mol
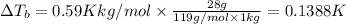
For NaI
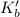 for water is = 0.59 K kg/mol
for water is = 0.59 K kg/mol
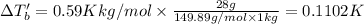
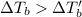
Since, all the values beside molar mass are same. The information of molar masses of KBr and NaI will be most useful to determine the solution that has the lower freezing point.