Answer:
8 moles of compound C can you produce with 2 moles of compound A and excess compound B.
Step-by-step explanation:
3A+5B → 12C
We are given with excess moles of compound B and 2 moles of compound A.
Moles of compound A = 2 moles
According to reaction, 3 moles of compound A gives 12 moles of compound C.
Then 2 moles of compound A will give:
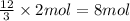 of compound C.
of compound C.
8 moles of compound C can you produce with 2 moles of compound A and excess compound B.