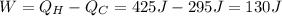Answer: 130 J
Step-by-step explanation:
For the law of conservation of energy, the total energy absorbed by the hot reservoir must be equal to the sum of the energy passed to the cold reservoir and the work done by the system:
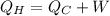
In this case, we have
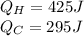
So, the work done by the system is