Answer: The correct answer is False.
Step-by-step explanation:
Decomposition reaction is defined as the chemical reaction in which a substance break down into two or more substances. The general chemical equation for the decomposition reaction follows:
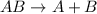
The products of decomposition reaction can be pure elements and compounds.
For Example:
- Decomposition of calcium carbonate to calcium oxide and carbon dioxide.
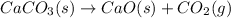
- Decomposition of water into elemental hydrogen and oxygen gases.
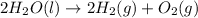
Thus, the correct answer is False.