Answer: The correct answer is synthesis reaction.
Step-by-step explanation:
Synthesis reaction is defined as the reaction in which two substances in their elemental state combine together to form a single molecule.
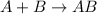
Combustion reaction is defined as the reaction in which hydrocarbon in the presence of oxygen decomposes to form carbon dioxide and water molecule.
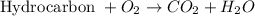
Single replacement reaction is defined as the reaction in which more reactive element replaces a less reactive element from its chemical reaction.
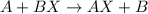
Double replacement reaction is defined as the reaction in which exchange of ions takes place.
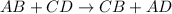
For the reaction given:
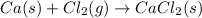
This reaction is a type of synthesis reaction because calcium and chlorine reacts to form calcium chloride.