Answer:
The correct answer is : 1 mol of
 = 44.01 grams of
= 44.01 grams of
 .
.
Step-by-step explanation:
Molar mass is defined as the sum of the mass of all the atoms each multiplied its atomic masses that are present in the molecular formula of a compound. It is expressed in g/mol.
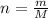
n = moles of compound
m = Mass of the compound
M = Molar mass of the compound
We are given with molar mass of carbon dioxide gas which is 44.01 g/mol.
The means that mass of the 1 mole of carbon dioxide gas is 44.01 grams.
n = 1 mole, m =?, M = 44.01 g/mol
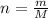
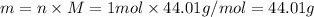
So , relationship between molar mass and mass of the carbon dioxide is given as:
1 mol of
 = 44.01 grams of
= 44.01 grams of
