Answer:
The correct answer is option A.
Step-by-step explanation:
Molarity is defined as the number of moles that are present in one liter of solution.
Mathematically,
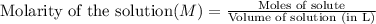
We are given:
Mass of barium chloride = 416.48 g
Moles of barium chloride =
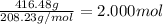
Molarity of solution = M = ?
Volume of solution = 2 L
Putting values in above equation, we get:
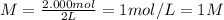
1 M is the molarity of 416.48 grams barium chloride dissolved in 2 liter of water.