Answer:
0.4155 is the mole fraction of oxygen gas in the mixture.
Step-by-step explanation:
Mole fraction is defined as ratio of moles of specific to the total moles of all the compounds present in the mixture.
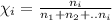
Moles of oxygen gas =
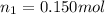
Moles of argon gas =
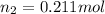
Mole fraction of oxygen gas =
=
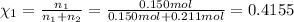
Mole fraction of argon gas =
=
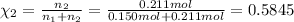
0.4155 is the mole fraction of oxygen gas in the mixture.
0.5845 is the mole fraction of argon gas in the mixture.