Explanation:
1) Charles's Law: This law states that the volume is directly related to the temperature of the gas when pressure and number of moles are held constant.
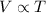 (At constant Pressure and number of moles)
(At constant Pressure and number of moles)
2) Boyle's Law: This law states that Pressure is inversely related to the volume of the gas when temperature and number of moles are held constant.
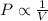 (At constant Temperature and number of moles)
(At constant Temperature and number of moles)
3) Avogadro's Law: This law states that Volume is directly related to the Number of moles of gas when Pressure and temperature are held constant.
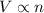 (At constant Pressure and Temperature)
(At constant Pressure and Temperature)
For the given options:
1) A balloon shrinks when it is taken outside in the winter.
In this the temperature is low outside in winters and as we take the balloon outside, the volume also as it shrinks. So, this situation follows Charles's Law.
2) When the size of an air chamber is increased, the air pressure decreases.
As the size is increasing which means that the volume is increasing and hence, the pressure is decreasing. This situation is following Boyle's Law.
3) A balloon expands when air is blown into it.
As the number of particles are increasing when we blow air into the balloon and as a result the size of the balloon is also increasing. Therefore, this situation is following Avogadro's Law.
4) A closed, flexible container expands when it is heated.
In this situation, the temperature is increasing and as a result thus container is also expanding. Thus, this situation is following Charles's Law.
5) Pressing on an inflated balloon decreases its size.
Here, the pressure is increasing the volume of the balloon is decreasing because the size is decreasing. This situation is following Boyle's Law.