Answer:
D. CH3CH2COO- for CH3CH2COOH; H2PO4 - for H3PO4.
Step-by-step explanation:
Hello!
In this case, since the ionization of a weak acid produces a conjugate acid, molecule to which the leaving H⁺ is added to, and a conjugate base, everything that remains after the withdrawal of the H⁺, we can note down the ionization of each acid down below:
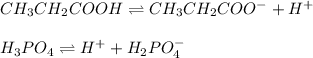
Thus, we notice that the answer is D. CH3CH2COO- for CH3CH2COOH; H2PO4 - for H3PO4 because the phosphoric acid is most likely to ionize to dihydrogen phosphate ions rather than phosphate, or hydrogen phosphate ions it undergoes a stepwise ionization in which the first step is the predominant one.
Best regards!