m = mass of the ice added = ?
M = mass of water = 1.90 kg
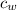 = specific heat of the water = 4186 J/(kg ⁰C)
= specific heat of the water = 4186 J/(kg ⁰C)
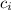 = specific heat of the ice = 2000 J/(kg ⁰C)
= specific heat of the ice = 2000 J/(kg ⁰C)
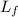 = latent heat of fusion of ice to water = 3.35 x 10⁵ J/kg
= latent heat of fusion of ice to water = 3.35 x 10⁵ J/kg
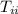 = initial temperature of ice = 0 ⁰C
= initial temperature of ice = 0 ⁰C
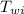 = initial temperature of water = 79 ⁰C
= initial temperature of water = 79 ⁰C
T = final equilibrium temperature = 8 ⁰C
using conservation of heat
Heat gained by ice = Heat lost by water
m
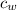 (T -
(T -
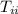 ) + m
) + m
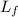 = M
= M
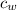 (
(
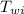 - T)
- T)
inserting the values
m (4186) (8 - 0) + m (3.35 x 10⁵ ) = (1.90) (4186) (79 - 8)
m = 1.53 kg