Step-by-step explanation:
Contributing structures are the resonating structures which are formed due to the delocalization of electrons in a molecule.
The azide ion that is
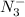 , is a symmetrical ion, all of whose contributing structures have formal charges.
, is a symmetrical ion, all of whose contributing structures have formal charges.
Lone pair of central nitrogen atom in azide ion is in conjugation with the neighboring nitrogen atoms.
Contributing structures of azide ion are drawn in the image attached.