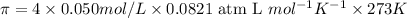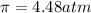Answer: Osmotic pressure of the given solution is 4.48 atm.
Step-by-step explanation: Osmotic pressure is defined as the number of moles of solute which are present in a solution.
Mathematically,
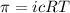 ....(1)
....(1)
where i = Van't Hoff factor
c = concentration of the solution
R = Universal gas constant =
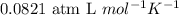
T = temperature of the solution
Value of 'i' is 1 for non-electrolytes. But, here
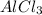 is an electrolyte, so the value of Van't Hoff factor will be the number of moles of particles of solute we get when 1 mole of solute is dissolved in a solution.
is an electrolyte, so the value of Van't Hoff factor will be the number of moles of particles of solute we get when 1 mole of solute is dissolved in a solution.
So, when 1 mole of
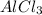 dissociates in aqueous state to produce 1 mole of
dissociates in aqueous state to produce 1 mole of
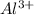 ion and 3 moles of
ion and 3 moles of
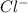 ion.
ion.
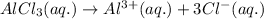
For
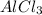 ,
,
i = 4
c = 0.050 mol/L
T = (273 + 0)K = 273 K
Putting the values in equation 1, we get