Answer: The
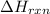 for the given chemical reaction is -175.51 kJ/mol
for the given chemical reaction is -175.51 kJ/mol
Explanation: Enthalpy change of the reaction is defined as the amount of heat released or absorbed in a given chemical reaction.
Mathematically,

We are given a chemical reaction. The reaction follows:




Enthalpy change for the reaction of he given chemical reaction is given by:
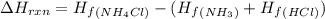
Putting the values in above equation, we get

