Answer:-
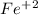
Explanations:- Transition elements shows variable oxidation states. So, while naming these compounds we use the roman numerals to tell their oxidation states.
For example, if someone writes just Iron oxide then it would not be correct since Iron could be in +2 or +3 states. So, it might be
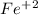 or
or
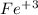 . So, the right was is to mention the oxidation state of the transition metal.
. So, the right was is to mention the oxidation state of the transition metal.
If the name is written as Iron(II) in a compound then it means there is +2 charge on iron and it is
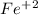 .
.