Answer: The correct answer is an
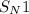 type reaction involving the protonated alcohol as the substrate.
type reaction involving the protonated alcohol as the substrate.
Step-by-step explanation:
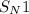 reactions are a type of nucleophilic reactions. These reactions are considered as the unimolecular reactions in which only 1 component is used to determine the rate of the reaction.
reactions are a type of nucleophilic reactions. These reactions are considered as the unimolecular reactions in which only 1 component is used to determine the rate of the reaction.
This reactions involved the generation of a carbocation as an intermediate.
For the reaction of 2-methyl-2-pentanol and HBr, the intermediate formed is protonated alcohol which yields the product 2-bromo-2-methylpentane.
Reaction is attached in the image below.